CT Imaging
Wake-up call for European stakeholders: CT lung cancer screening approved by CMS
Tools from MeVis Medical Solutions AG support radiologists in implementing screening programs
The U.S. Centers for Medicare and Medicaid Services (CMS) have approved CT lung cancer screening for high-risk individuals. This decision clears the implementation path of screening for the general public. Solutions supporting imaging centers in this complex process are showcased at RSNA 2014.
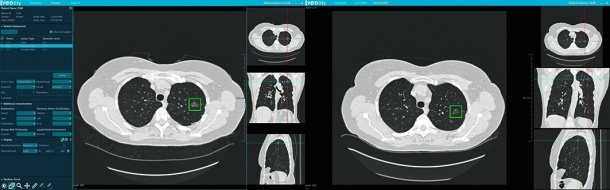
Lung cancer is the leading cancer killer. It causes more deaths than colon, breast, and pancreatic cancer combined. The prime challenge is that patients often present only when their condition is symptomatic – at a stage where treatment has become difficult. Results of the U.S. National Lung Screening Trial (NLST) show a 20 percent reduction in mortality due to early detection provided by CT screening. Even though European studies like the Dutch-Belgian “NELSON” are still being evaluated, it is expected that European countries will follow in the near future. The decision of the CMS may well contribute to the accelerated adoption of lung screening.
With the CMS decision, lung screening will become available to over 8 million Americans. Providers of imaging services will have to overcome the challenge of handling the related workload. Precise reading tools, standardized reporting and efficient workflows will be needed to ensure diagnostic precision and cost effectiveness.
With Veolity, the medical software specialist MeVis Medical Solutions AG in Bremen/Germany, provides a dedicated workstation for lung cancer screening. Based on leading-edge research, Veolity optimizes reading workflows. It brings together lung CAD – computer-aided detection – of solid pulmonary nodules approved by the FDA, integration and automatic registration of prior studies, and efficient, state-of-the-art reporting. Reports are generated automatically according to current standards and guidelines, including current models for nodule malignancy prediction. Automated measurements, integration of incidental findings, determination of patient follow-up, and integration of screening worklists make the system a complete solution for reading lung screening CT data.
“Making CT lung screening available to high-risk population will be saving thousands of lives every year. We highly welcome this decision, not only because it sparks additional interest in our products, but mainly because of the tremendous benefit for the patients.”, explains Bernd Kuemmerlen, Product Manager, MeVis Medical Solutions AG. “With Veolity, we aim to make the workload for the radiologists manageable, while at the same time improving quality and thus the benefit for the patient. Finding, measuring and comparing nodules to prior exams manually is tedious work. Veolity automates most of these steps, ensuring diagnostic precision by detecting nodules, providing objective measurements, and comparing with prior findings.”
RSNA 2014: Meet the experts
MeVis will showcase solutions for radiologists at booth 2965-F in hall A, South Building, German Pavilion. From Sunday through Tuesday, renowned experts will be available to discuss current approaches to lung screening and solutions available to support them.
Schedule: Meet the experts at RSNA 2014!
More Information:
MeVis Medical Solutions AG
Martina Hallmann
Public Relations
Caroline-Herschel-Str. 1
28359 Bremen, Germany
Phone: +49 421 22495-301
E-mail: martina.hallmann@mevis.de
01.08.2014


