Sponsored • Standardization
MRI safety in practice
Missing standardized MR labeling information – related to EN IEC 62570 “MR Safe” / ”MR Conditional” labeling requirements for medical devices and MR accessories – endangers MR user and MR patient safety.
Commercially available accessories such as furniture, wheel chairs, instruments, gas cylinders, etc. can be ferromagnetic or electrically conductive. Those are not designed, thus contraindicated to be used in the MR environment (MRE).
Several health injuries have been reported by use of incorrect or unlabeled MR devices. EN IEC 625701 standards address “MR Safe” / “MR Conditional” marking and identification of test requirements for all items with intended use inside the MRE. After technical training, the MR personnel, MR authorized person or MR worker must learn the terminology of the MR environment and how it is applied. MR user education for MR safety is offered by training courses as “MR safety expert (MRSE)”, www.mrcomp.com/mr-education.html, Germany, DIN 68762 and OENORM 1125-1/-2 at AHK Wien.
Relevant MR interactions to consider:
MR safety
- Magnetically-induced displacement force exist for devices consisting of ferromagnetic materials
- Magnetically-induced torque aligns the device to the orientation of the field
- Gradient- & RF-induced heating / voltages
- Gradient-induced vibration
- Malfunction induced by all three fields
MR compatibility
- Image artefacts: can distort or misplace image information.
- Amongst further image quality interferences disturbing the MR image quality are e.g.:
- B0-inhomogeneities by ferromagnetic masses
- Eddy currents by induced currents in electrically conductive components
- RF noise emitted from unshielded accessories
- Proton signals generated by hydrogen protons in plastics
More sophisticated electrical/active devices need additional consultation of ISO/TS 10974 test methods. Implant MR safety labeling can be found in MR safety implant databases such as www.MagResource.eu
96 commercial available MRI products (www.MRI-tec.com, one-shop-stop, Germany) have been selected randomly and from throughout the daily use of MR clinical application:
- Audio and video systems
- Gurneys
- Goggles
- Injection systems
- Suction pumps
- Monitoring system
- Positioning
- Wheel chairs
- Anesthesia machines
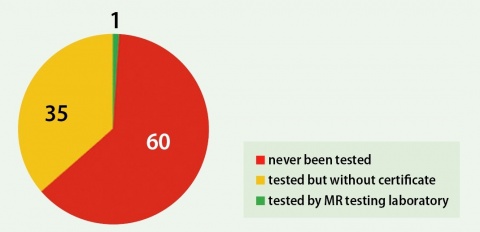
The product documentation has been investigated for any existing MR labeling and the completeness of it. From 96 Products that have been analyzed, more than half of the investigated products have never been properly tested and assessed for safety in the MR environment.
There could be fatal consequences if products contain ferromagnetic materials, be conductive, thus hurt the patient or have its function affected as well as disturbing the MR system, if a device is not fully tested. Individual MR Statements of manufacturers lead to caution, but cannot be considered as being sufficient for use in the daily clinical MR routine due to many factors resulting in unclear situations.
Comprehensive testing of all MR interactions is necessary and is only given by the international standards, which have reached nowadays a useful and comprehensive quality level. Only then MR user and MR patient safety is guaranteed.
www.mri-tec.com
www.mrcomp.com
03.03.2017


