Liquid biopsy
It sounds amazingly simple: a structured medical Seldinger guidewire is inserted via a peripheral venous catheter to ‘fish’ for circulating tumour cells (CTC) in the blood of cancer patients.
Report: Bettina Döbereiner
The new technology called Gilupi CellCollector has a unique feature – its high degree of sensitivity. Since the isolation of the CTCs is done in vivo a large sample volume of up to 1000 ml blood can be used for screening purposes – which was impossible even with the current gold standard for cell isolation, the CellSearch technology. EH correspondent Bettina Döbereiner, spoke with Dr Claudia Chudak of Gilupi, the firm that developed and markets the system.
Circulating tumour cells (CTC) are the objects of intensive research because their detection is considered crucial for the diagnosis and therapy of solid epithelial tumours.
What we do know about CTCs is their prognostic value as a biomarker: the CTC count per millilitre blood provides data on a potential metastatic relapse and cancer progression and thus on overall survival. Moreover the CTC count is a monitoring device to evaluate therapy response and molecular analysis of the CTCs offer insights into the molecular ‘ductus’ of the primary tumour and the metastases. In short: the CTCs fully map the tumour’s heterogeneity. This is particularly important for the so-called targeted cancer therapy since mutations or resistances that have developed during the therapy can be detected quickly and medication can be adjusted accordingly.
The ‘fishing for CTCs’ procedure, also called liquid biopsy, has several advantages as Dr Claudia Chudak, marketing and sales specialist at Gilupi, explains: it is minimally invasive if you consider drawing blood an invasive procedure; it can be repeated and performed at any time.
The only true challenge to be mastered is the actual detection of CTS, since very few of them actually circulate in the blood. Scientists estimate there is one CTC on 107 white blood cells in one millilitre of blood. The current gold standard to isolate CTCs – the US-American product CellSearch – is an in vitro procedure and can therefore be performed on a sample of at most 7.5 ml, the standard blood-taking sample.
Gilupi CellCollector, which received CE approval in 2012, has two major advantages compared to CellSearch says Dr Chudak: the CTS are isolated in vivo instead of having to be separated from the other blood components in a complicated ex vivo procedure. The crucial advantage, however, is the fact that the CellCollector can screen up to 1000 ml of blood in the 30-minute procedure.
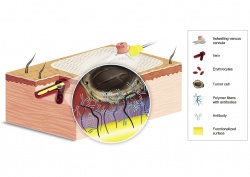
The technology itself is straight forward: the Gilupi CellCollector, which is inserted via a peripheral venous catheter in the cubital vein, features a rounded tip with a 2 cm long layer of pure gold. The gold layer is coated with a functionalised polymer, which in turn is covalently bonded with anti-EpCam antibodies. They dock onto the epithelial cell adhesion molecule EpCam, which is present in most CTS (see Figure 1). Studies have shown that the system can isolate CTS in more than 70% of all patients diagnosed with cancer of the lung, breast, prostate or colon. Further studies, including head/neck, renal and neuroendocrine tumours, are being conducted.
The CTCs detected by the Gilupi CellCollector undergo simple post-processing and can then be analysed using the standard microscopic or molecular diagnostic procedures (see Figure 2).
According to Dr Chudak liquid biopsy is significant for targeted cancer therapy because it offers a good and easy to use option to map the status of the primary tumour and metastases. It is particularly useful when a conventional biopsy is too dangerous, or when the primary tumour has already been removed. A molecular analysis of the CTS can also provide early information on mutations and resistances that have developed over the course of the therapy. Thus, second or third line medication can be designed more efficiently.
To date the sole limitation of the Gilupi technology is the fact that not all CTCs can be detected in the blood since not all of them feature the protein EpCam at all, or in a sufficiently expressed form such as mesenchymal or anaplastic phenotypes. However, this limitation applies not only to the Gilupi technology but also to most technologies to isolate CTCs, including the above-mentioned CellSearch.
Dr Chudak hopes that advances in antibody research will allow the tip to be fitted with additional antibodies or substances to detect non-EpCam positive CTCs.
The Gilupi CellCollector is not limited to cancer applications, he adds: it is considered to be a technology platform for the detection of a wide variety of targets in the blood, for example bacteria. Indeed, the inventors Professor Michael Giersig, Dr Klaus Lücke and Professor Ulrich Pison, whose initials of their last names form the company name, had initially designed the cell-collecting device for prenatal diagnoses, as an elegant alternative to the dangerous amniocentesis. While the production of that device was stopped due to lack of demand, the scientists at this medium-sized enterprise have developed two further prototypes – for cardiology and intensive medicine.
In cardiology the Gilupi CellCollector is intended to detect circulating endothelial cells that provide reliable information on the presence of acute micro-infarcts (AMI), which the current standard test for troponin cannot always do. In intensive medicine, in vivo cell enrichment is supposed to quickly detect infections or rather their specific pathogens. This can, for example, facilitate the recognition of sepsis.
* Ref: Nadia Saucedo-Zeni et al. A novel method for the in vivo isolation of circulating tumour cells from peripheral blood of cancer patients using a functionalised and structured medical wire, International Journal of Oncology, 2012 (DOI: 10.3892/ijo.2012.1557).
Profile:
Dr Claudia Chudak, marketing and sales specialist at Gilupi GmbH, studied biology at Free University Berlin, Germany. She became a junior researcher on HIV and other retroviruses at Robert Koch Institute in Berlin and wrote her dissertation of the identification and functional characterisation of L-domains of the human endogenous retrovirus K.
21.11.2014


