CT Imaging
Fast, safe, and convenient
Veolity by MeVis Medical Solutions AG supports high-volume CT-based lung screening
RSNA 2014: With lung screening approved by the CMS and endorsed by the USPSTF, MeVis Medical Solutions AG helps imaging centres handle the vast number of clients: Veolity, a reading platform for high-throughput environments, supports radiologies in ensuring diagnostic precision and safeguarding adherence to guidelines.

Lung cancer is the number one cancer killer in the United States. The National Lung Screening Trial had proved that CT lung cancer screening (LCS) can reduce deaths due to the disease by at least 20% in individuals at high risk. The resulting endorsement of LCS by the United States Preventive Services Task Force (USPSTF) means that millions of high-risk Americans will be granted insurance coverage for annual LCS beginning in 2015. In addition, the Centers for Medicare and Medicaid Services (CMS) recently approved CT lung cancer screening for a large number of U.S. citizens covered by Medicare and Medicaid. Hundreds of CT lung screening programs will be required throughout the United States to meet this oncoming demand. European health systems are expected to follow suit.
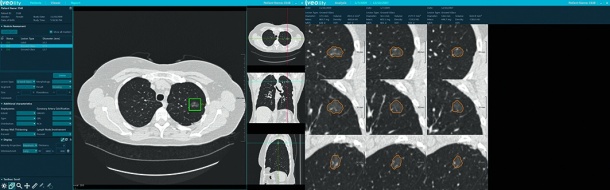
The challenge to be met by providers of imaging services is manifold, ranging from handling enormous numbers of patients and ensuring diagnostic precision to safeguarding adherence to guidelines. Veolity, a reading platform for high-throughput environments developed by medical software specialists MeVis Medical Solutions AG in Bremen/Germany, supports radiologies in achieving these aims.
Based on leading-edge research, Veolity optimizes reading workflows. It brings together lung CAD – computer-aided detection – of solid pulmonary nodules approved by the FDA, integration and automatic registration of prior studies, and efficient, state-of-the-art reporting. Reports are generated automatically according to current standards and guidelines, and nodule malignancy prediction is added to reports. Additional features include automated measuring, integration of incidental findings, determination of patient follow-up, and integration of screening worklists.
“Reading used to be a cumbersome process full of risks”, explains Bernd Kuemmerlen, Product Manager, MeVis Medical Solutions AG: “Radiologists, who are subject to enormous workloads, need to analyze hundreds of slices to identify nodules of which there may be several in an individual case. They have to measure those nodules manually and write their report. Veolity automates most of these steps, ensuring diagnostic precision by detecting nodules, by providing objective measurements, and comparing results with prior findings. In the U.S. as well as in Europe, there is widespread interest in Veolity from organizations which prepare themselves for the launch of lung screening programs.”
RSNA 2014: Meet the experts
MeVis will showcase further solutions for radiologists at booth 2965-F in hall A, South Building, German Pavilion. From Sunday through Tuesday, renowned experts will be available to discuss current approaches to lung screening and solutions available to support them.
Schedule: Meet the experts at RSNA 2014!
More Information:
MeVis Medical Solutions AG
Martina Hallmann
Public Relations
Caroline-Herschel-Str. 1
28359 Bremen, Germany
Phone: +49 421 22495-301
E-mail: martina.hallmann@mevis.de
01.08.2014


