Video • LIMA project
Agena Bioscience’s liquid biopsy technology awarded Horizon 2020 grant
Agena Bioscience, a global provider of molecular genetic solutions, announced it has been selected to participate as an innovative technology provider in the ‘Liquid biopsies and IMAging for improved cancer care’ (LIMA) project.
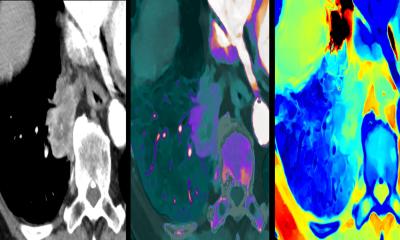
The LIMA project has been awarded a EUR 6.3 million Horizon 2020 European Union research grant to develop an integrated approach for personalized cancer treatment. The objective of the four-year project is to develop and validate liquid biopsy technologies in the clinical workflow and to provide more precise, dynamic tumor characterization during diagnosis and treatment. Agena Bioscience’s MassARRAY System will be used to provide molecular genetic information from liquid biopsies. In collaboration, advanced Magnetic Resonance Imaging (MRI) from Royal Philips will be utilized for in-vivo tumor characterization.
Liquid biopsy is an emerging field of oncology diagnostics where disease prognostic and resistance markers are identified from circulating tumor DNA and cells present in blood. Liquid biopsies are non-invasive, provide a more accurate picture of the disease’s heterogeneity, and are repeatable at regular intervals to monitor the diseases’ status and response to treatment.
We are excited to participate in the LIMA project as a subject matter expert for the characterization of liquid biopsies
David Coorey
The UltraSEEK chemistry on the MassARRAY System enables detection of over 50 variants from a single blood draw, as low as 0.1% variant allele frequency. The simple workflow generates results in as fast as a day, enabling minimal residual disease and resistance monitoring.
“We are excited to participate in the LIMA project as a subject matter expert for the characterization of liquid biopsies,” commented David Coorey, Vice President & Managing Director, EMEA at Agena Bioscience. “The MassARRAY System’s low limit of detection along with advanced MRIs could provide better genotypic and phenotypic characterization of the disease, helping physicians make more informed personalized treatment decisions, thereby improving patient care.”
The MassARRAY Dx is CE-IVD marked for use in Europe Only. The MassARRAY System and UltraSEEK are for Research Use Only. Not for use in diagnostic procedures.
Source: Agena Bioscience
01.12.2017