Article • Early warning
Blood test detects early Alzheimer’s
The cause of Alzheimer’s disease remains unknown. Early diagnosis is currently not possible as clinical symptoms only occur once a large number of neurones in the brain have already been destroyed. There is no treatment available either.
Report: Melanie Günther
The advantage of our technology is that we can determine the secondary structure distribution of all amyloid-beta peptides extracted from body fluids label-free.
Prof. Klaus Gerwert
Imaging procedures such as PET-MRI are expensive and not covered by routine care. This situation hinders the early confirmation of a diagnosis. A new blood test now makes it possible to diagnose Alzheimer’s up to 20 years before the onset of the first clinical symptoms. Amyloid-beta peptides, i.e. proteins, and an infrared sensor, play key parts in this process. In our interview, Professor Klaus Gerwert, Head of the Chair for Biophysics at the Ruhr University Bochum, Germany, discusses the procedure.
‘There are different hypotheses that describe how Alzheimer’s develops, but none of these has been conclusively proven,’ explained biophysics expert Professor Klaus Gerwert, when asked about the role of amyloid-beta peptides in Alzheimer’s disease. ‘We assume that, at the beginning of the disease, there’s a change in the amyloid-beta peptide. This means that the peptide changes from a healthy alpha-helical form into a diseased, beta-pleated sheet form; the secondary structure of the protein changes. The pleated sheet structures can attach to one another and form oligomers. We believe that the formation of soluble oligomers is the critical point at the beginning of Alzheimer’s disease. The oligomers develop into fibrils and finally into visible plaques.’
Along with other scientists, Gerwert has developed a test to detect change in amyloid-beta peptides and thus diagnose Alzheimer’s earlier.
How does the test, or procedure, work?
We utilise antibodies as ‘interceptors’ to specifically bind amyloid-beta peptides
Prof. Klaus Gerwert
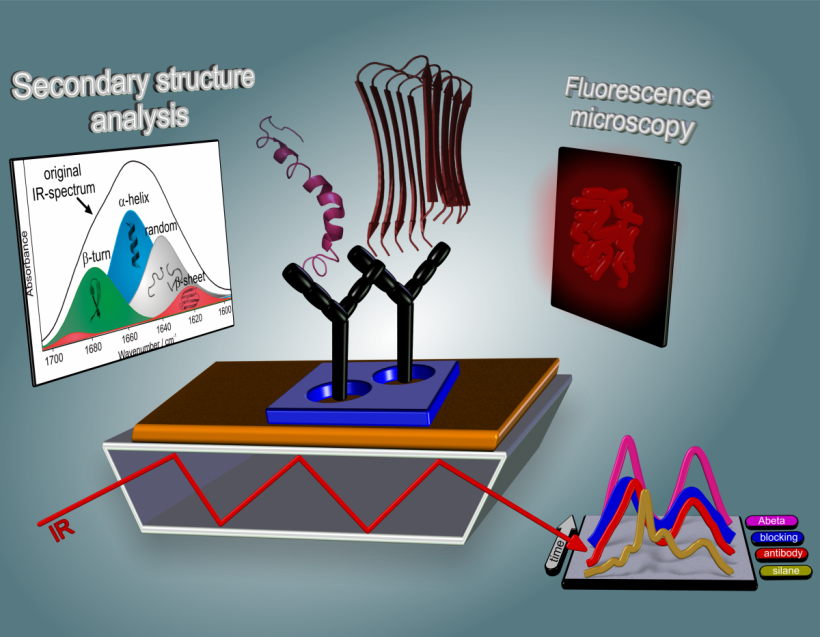
‘We have developed an infrared sensor. This detects the secondary structures and differentiates between alpha-helix and beta pleated sheet forms. However, various different types of proteins are found in the blood or spinal fluid. The difficulty is being able to look only at the amyloid-beta. Other substances impair this and interfere with the actual signal. We utilise antibodies as ‘interceptors’ to specifically bind amyloid-beta peptides. These are covalently bonded to the surface of the infrared sensor, meaning that we have a type of ELISA-test (Enzyme-Linked-Immunosorbent Assay).
‘We then measure the bonded amyloid-beta peptide with the infrared sensor and analyse whether it is present in its healthy or diseased form. We utilise structure-sensitive infrared bands, i.e. so-called amide 1 bands. When these bands are below a certain threshold we know that Alzheimer’s disease is present. ‘We determined the threshold level based on experimental investigations.’
What does the importance of amyloid-beta peptides distribution mean?
‘This is the key part of the test: There are antibodies that can only selectively bind one form of amyloid-beta peptides – either the healthy or diseased form. The blood of a healthy human contains a large number of healthy amyloid-beta peptides, but naturally also some diseased ones. An antibody that only selectively looks for the beta pleated sheet form would therefore also find diseased forms in a healthy person. Therefore the test is initially confusing.
‘The advantage of our technology is that we can determine the secondary structure distribution of all amyloid-beta peptides extracted from body fluids label-free. When the peptides are healthy we can see alpha-helical dominated bands above the threshold level. When the majority of peptides are of the beta pleated sheet form, the band goes below the threshold level. Unlike the ELISA procedures we measure the distribution of all amyloid-beta peptides and not only specific conformations.
Early detection timescale
There are different hypotheses that describe how Alzheimer’s develops, but none of these has been conclusively proven.
Prof. Klaus Gerwert
‘Our primary objective was to detect an early stage of Alzheimer’s, i.e. before the onset of clinical symptoms. Therefore, we selected the amyloid-beta peptide, because the change occurs around 15 – 20 years before the beginning of clinical symptoms.
‘We’ve already carried out a mini-study which, although not yet statistically significant, has delivered some promising results. We analysed samples from a cohort from the year 2000, at the Heidelberg-based German Cancer Research Centre (DKFZ). We could analyse, conclusively, which of the initially healthy study subjects developed Alzheimer’s disease within the following 8-15 years. To substantiate our analysis with statistical accuracy, we are currently examining 1,000 samples from study participants. Once we can make this prediction for all 1,000 samples we’ll have achieved the necessary statistical significance. So far we have tested 300 out of the 1,000 samples.’
Other neurodegenerative disease
‘The sensor is also potentially suitable for the detection of Parkinson’s disease. In the case of Parkinson’s the so-called alpha-synuclein plays an important role. We assume that this protein also converts from an alpha shape to a beta shape. We are currently looking for a specific antibody for alpha-synuclein, allowing us to use the sensor for the detection of Parkinson’s disease.’
When might the test enter clinical routine?

‘The procedure is very robust in the laboratory. It is suitable for the detection of Alzheimer’s. It is a good, additional clinical-chemical test to confirm an Alzheimer’s diagnosis. ‘We need to await the results of the current study to assess its use for early detection. We are currently optimising the sensor and are trying to increase the sample throughput so that large collectives can be measured in short periods of time.’
Profile:
Professor Klaus Gerwert is Head of the Chair for Biophysics at the Ruhr University Bochum. As Chair of Biophysics, the professor. and his collegues, investigate structure, function and interaction of proteins at the atomic level, using a wide variety of interdisciplinary methods from biology, biophysics, biochemistry and computing. In the post-genome-era the focus of research has moved more towards proteins, which are, besides DNA, key players on a molecular level.
25.07.2016