News • Oncology
Tumor blood vessel a target to treat glioblastoma?
A novel protein regulator of tumor angiogenesis, TMEM230, was recently characterized by researchers to have a role in tumor development and vascularization, with potential as a target for anti-tumor therapy in difficult-to-treat cancers such as glioblastoma.
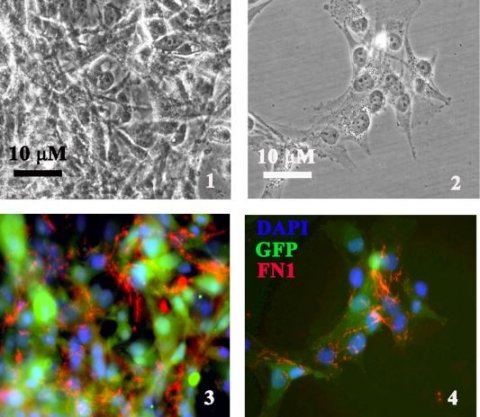
TMEM230 increases tissue vascularization by 3 mechanisms: inducing tumor cell migration and intussusceptive remodeling of existing blood vessels; promoting secretion of proangiogenic factors; favoring tumor cells generating microchannels, a process described as vascular mimicry.
“These findings provide insight into why anti-angiogenic therapies are often ineffective in certain types of highly vascularized tumors,” explains lead author, Dr. Cinzia Cocola, of the Institute of Biomedical Technologies of the Consiglio Nazionale delle Ricerche (ITB-CNR) in Milan, Italy.
The paper is a result of collaboration between researchers from Italy, Germany and the United States, led by Cocola, with co-authors Dr. Valerio Magnaghi, Dr. Edoardo Abeni, and Dr. Ileana Zucchi.
The TMEM230 gene initially was identified in regulating normal blood vessel formation in early development by Dr. Paride Pelucchi at the Institute of Biomedical Technologies of the ITB-CNR. Of particular clinical interest is that TMEM230 functions independently of canonical angiogenic signaling pathways, as initially determined in zebrafish models in collaboration with Prof. Franco Cotelli at University of Milan and Prof. Gianfranco Bellipanni at the Sbarro Institute for Research and Molecular Medicine, Temple University. This latest study builds on earlier findings to explore TMEM230 as a therapeutic target where standard anti-angiogenic treatments have previously failed.
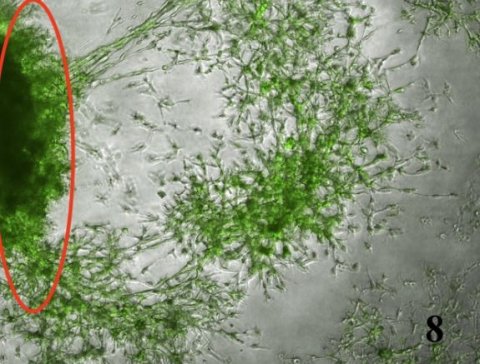
Precise levels of TMEM230 determine normal blood vessel homeostasis and blood vessel formation in early development or normal wound healing. Aberrantly elevated expression levels of TMEM230 result in cells developing aggressive tumor properties associated with a tumor angiogenic switch that may lead to metastasis. The identified cell function is supported by gene expression analyses of patient derived glial tumors, performed by Dr. Edoardo Abeni of the ITB-CNR.
Dr. Valerio Magnaghi of the Department of Pharmacological and Biomedical Sciences at the University of Milan, Italy, who studies glial cells, states that our data supports that progression from low grade glioma to glioblastoma is regulated in part by TMEM230.
Insight into the various diverse roles that TMEM230 plays are being identified using an animal model system in the lab of Dr. Daniela Marazziti from the Institute of Biochemistry and Cell Biology at the CNR in Monterotondo, Rome.
“We believe TMEM230 has clinical therapeutic applications both in vascular disease and regenerative medicine,” say the clinician collaborating authors working in Italy and Germany, Dr. Daniela Mazzaccaro and Prof. Giovanni Nano of the Operative Unit of Vascular Surgery at the IRCCS Policlinico of San Donato, and Dr. Martin Götte and Dr. Mira Palizban of the Klinik für Frauenheilkunde und Geburtshilfe, Universitätsklinikum Münster.
Dr. James Kehler, SVP of Translational Research at Mirimus Inc. in Brooklyn, believes TMEM230 has all the properties of a master regulator in normal and disease blood vessel development. Dr. Ileana Zucchi, of the ITB-CNR, emphasizes that glioblastoma is the most aggressive tumor originating in the brain and as abnormal blood vessels contribute to the inability to direct therapeutic agents to tumor cells, TMEM230 therefore is an attractive therapeutic target. Identification of novel native targets remains an important goal for effective treatment of highly vascularized tumors such as GBM, as inhibition of endogenous gene expression may be less toxic and less likely to lead to drug resistance than small molecule or antibody-based therapies.
Funding of the research was provided by the “Progetto di Eccellenza” from the Ministry of Research to Dr. Valerio Magnaghi and by CNR-MIUR Flag Projects Epigen and Interomics to Drs. Ileana Zucchi and Rolland Reinbold.
Professor Antonio Giordano MD, PhD, President of the Sbarro Institute for Research and Molecular Medicine, highlights the importance of the collaborations between USA, Italy and Germany to identify new potential molecular targets for understanding the mechanisms of very aggressive cancer types. Director of the ITB-CNR, Dr. Gianluca De Bellis emphasizes that a major aim of the ITB-CNR, Milan Italy is to foster international collaborations in clinical medicine and biotechnology.
Source: Sbarro Health Research Organization (SHRO)
25.11.2021